We firmly believe in the fact that the inhalation preparation is and will be the mainstream product in the market.
Our products will be approved in China and EU from year 2021 and on, and with a global sell, we will be a leading company in inhalation products and eyedrops soon.
Warm Congratulations to Nanjing Aureole for Its Exclusive Made-in-China Inhalation Solution Dingkang® Included in China’s National Reimbursement Drug List in 2023
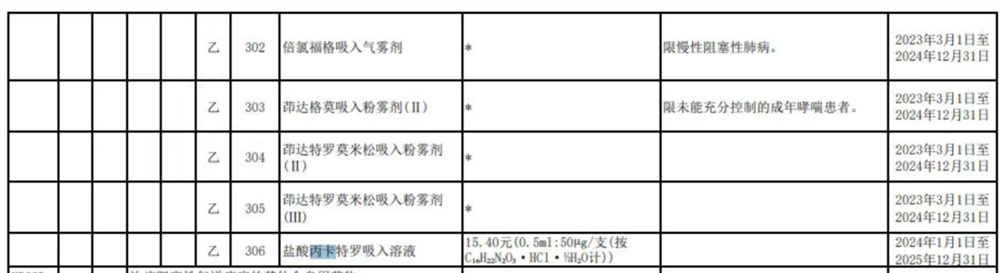
On December 13, 2023, the List of Drugs Covered by China’s Basic Medical Insurance, Work-related Injury Insurance and Childbirth Insurance (2023) (hereafter referred to as “China’s National Reimbursement Drug List”) was unveiled by China’s National Healthcare Security Administration and Ministry of Human Resources and Social Security, to officially release the list of drugs that passed the negotiation on drugs to be covered by the national medical insurance system. The product Dingkang® (Procaterol Hydrochloride Inhalation Solution) exclusively developed by Nanjing Aureole Pharmaceutical Co., Ltd. (hereafter referred to as “Nanjing Aureole”) passed the negotiation, and has been included in China’s National Reimbursement Drug List.
Currently, Salbutamol is the first aid medicine commonly used in clinical practice for treatment of patients with asthma or chronic obstructive pulmonary disease. Salbutamol is a racemate easy to induce adverse reactions such as tachycardia, hypokalemia, and even acidosis in case of serious conditions. The Procaterol launched can not only reduce the risk of the above adverse reactions, but also significantly improve patients’ pulmonary function, lower the risk of outpatient admission and readmission, and shorten hospitalization, providing patients with a new therapy with better safety and efficacy.
Inhalation product is a typically complex drug product, and is researched and developed preferentially with the encouragement and supporting of the state by virtue of its various advantages in clinical treatment. The market for this field in China is rising rapidly, showing broad developing prospects. Due to the high barriers to the research and development and manufacture of inhalation products and the great difficulty in imitation, the vast majority of the market share of inhalation products in China has been occupied by a few multinational pharmaceutical companies from Europe, the United States or Japan. Nanjing Aureole responds to the call of the state, actively performs the layout of respiratory system drugs, and focuses on breaking through the high-tech barriers to the research and development and manufacture of inhalation products, breaking the monopoly of multinational pharmaceutical companies. The product Dingkang® (Procaterol Hydrochloride Inhalation Solution) of Nanjing Aureole has been one of the Class 3 inhalation products newly marketed with exclusive approval in China, and is also the only made-in-China inhalation product for bronchiectasis included in China’s National Reimbursement Drug List. According to public data, Nanjing Aureole obtained the approval letter for the registration of Dingkang® (Procaterol Hydrochloride Inhalation Solution) in June 2023. The inclusion of Procaterol Hydrochloride Inhalation Solution in China’s National Reimbursement Drug List further demonstrates Nanjing Aureole’s high attention to the research and development, manufacture and application of inhalation products, as well as the recognition of its products and technologies from the state and pharmaceutical industry.

Nanjing Aureole Pharmaceutical Co., Ltd. is one of the companies in China to actively carry out the layout of inhalation products in recent years, and has been at the leading level in China in both R&D investment and pipeline. With the main keynote of scientific research and innovation proposed by the Chairman Sun Xiangyang, the Company always adheres to take the inhalation administration technology platform and innovative research and development technology platform as a breakthrough, to carry out in-depth layout in the field of inhalation products. With years of constant development, the Company has formed a relatively focused and clear product pipeline by now.
For every industry, it is vital for the development of enterprises to enhance scientific research and innovation capabilities and master core technologies in key fields. Nanjing Aureole dives deep into the pharmaceutical industry, makes breakthroughs constantly, and is always bold in making innovations. Beside breaking the monopoly of inhalation products by foreign countries, the Company also provides many patients with more high-quality and low-price drugs.

